This follows a previous article with a general summary of the two medication studies for BPD presented here: http://pdan.org/drug-trials-encouraging-results-for-bpd/
SEROQUEL XR (OLANZAPINE) STUDY FOR BPD, 2010-2013
“Comparison of Low and Moderate Dosages of Extended- Release Quetiapine in Borderline Personality Disorder: A Randomized, Double-Blind, Placebo-Controlled Trial”, by Donald Black, Mary Zanarini, Ann Romine, Martha Shaw, Jeff Allen, and Charles Schulz. Am J. Psychiatry (Nov. 2014) Vol. 171, No. 11, pp 1174-1182.
http://ajp.psychiatryonline.org/…
The study looked at about 100 patients suffering from BPD. This study did not use anypsychotherapy. It researched the changes in severity of traits or symptoms of BPD along certain psychological scales.
The study took place from January 2010 to March 2013. It followed these patients through an 11-week period for each patient. The first 2 weeks consisted of office visits for screening and to assess the mental state of these patients, followed by 8 weeks of medication (without psychotherapy), during which the patients were seen once a week by a professional to assess their progress, followed by an 11th week office visit to assess their resulting mental and physical state.
After the initial interviews, 95 patients were qualified for the medication phase of the study. The patients were between the ages 18 and 45, living in the USA, with levels of moodiness, impulsivity, distrustfulness and difficult relationships, which satisfied the DSM criteria for BPD.
These 95 patients were randomly placed into three groups that were given certain dosages of Quetiapine extended-release, known to consumers as Seroquel XR.
A placebo is a sugar pill. The act of just taking a pill can make people feel better because they think the pill is helping them. in general, for almost any mental condition, it has been observed that about 30 percent of people in a study report feeling better if they thought they were taking real medication, even though it was a sugar pill. That is why researchers use a “control group” of people: to determine if the results are from the pill or in people’s mind.
Neither the doctors administering the medication, nor the patients knew who was taking a sugar pill and who was taking the real medication. This is called a “double blind” study, and is the gold standard to make sure the results come from the medication only.
Patients in the Low-Dosage Group received 50 mg/day the first week; then 150 mg/day for 7 weeks.
Patients in the Moderate-Dosage Group received 50 mg/day the first week, 150 mg/day for 3 weeks, then 300 mg/day for 4 weeks.
People in the third group received placebo pills containing no active medication.
The group sizes at the beginning of the study were:
- Placebo (control) Group: 29 persons
- Low-Dosage Group: 33 persons
- Moderate-Dosage Group: 33 persons
By the 11th week final assessment, 64 patients completed the study out of the 95 initial patients, for a retention rate of 67%.
The group sizes at the end of the study were:
- Placebo (control) Group: 23 persons
- Low-Dosage Group: 22 persons
- Moderate-Dosage Group: 19 persons
The overall completion rate for the 8-week double-blind treatment phase was 67% (67% for the low-dosage quetiapine group, 58% for the moderate-dosage quetiapine group, and 79% for the placebo group). Participants who experienced sedation were more likely to drop out.
Among participants who completed the study, 82% in the low-dosage quetiapine group were rated as “responders,” compared with 74% in the moderate-dosage group and 48% in the placebo group.
Treatment-emergent adverse events included sedation, change in appetite, and dry mouth.
Several diagnostic scales were used to study the severity of various symptoms throughout the duration of the clinical trial. The main scale used was the Zanarini-BPD scale (Z-scale). This scale looks at patients along nine dimensions (the 9 criteria of BPD) and asks the person filling in the questionnaire (the patient or therapist, both evaluations were used in the trial) to evaluate the severity of their symptoms on a scale of 0 to 4. Then one adds up the score in each dimension. Hence the total score can vary from 0 to 36.
Normal Level on Zanarini-BPD Scale
When one takes the Zanarini-BPD scale questionnaire, a “normal person” might give herself or himself a score of 0 to 2 in a few dimensions. In another study comparing BPD patients to psychiatric patients without BPD, those without BPD had a score of 5.2 on the Zanarini-BPD scale.
Clinical BPD is considered a total Z-scale score of 9 or greater on the scale. Most BPD treatment studies start with a population of people with initial scores around 16 to 19. After treatment, scores may fall to around 9-13 depending on the study. For BPD patient, anything under 9 would be considered a very substantial improvement.
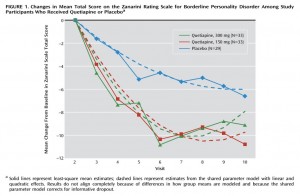
RESULTS ON Z-SCALE FOR BPD
In this Seroquel XR study (table 2), the initial mean Z-scales of
- Placebo group : 14.6
- Low dosage : 15.8
- Moderate dosage: 17.7
At the end of the trial, researchers from a decrease of
- – 6.0 for Placebo
- – 9.8 for Low dosage
- – 7.9 for Moderate dosage
Thus, the end mean results were:
- Placebo: 8.6
- Low dosage: 6.0
- Moderate dosage: 9.8
In other words the Low-dosage group achieved by the end of the trial – on average for the group – remission from clinical BPD on the Z-scale. This is a rather remarkable feat for a group of 22 people.
The Moderate-dosage group achieved by the end of the trial a level that was near normal, below 10, though still above 9. A Z-scale level of 9 or greater is viewed as clinical BPD. Note that this group started with an average Z-scale that was almost 2 points higher than the Low-dosage group, hence their average decrease on the scale was impressive too.
A Z-scale level of 6.0 is also much closer to the level of 5.2 found in non-BPD patients in another study. Charles Schulz remarked: “There is a study comparing the BPD patients to psychiatric patients without BPD. Those without BPD have a score of 5.2 and the BPD patients in the low dosage group had a score of 6.0. So in our Seroquel XR study, at a Zanarini-scale of 5.2 the Low-dosage patients were very close to non-BPD people.”
Study: Mary C. Zanarini, (2003). Zanarini Rating Scale For Borderline Personality Disorder (ZAN-BPD): A Continuous Measure of DSM-IV Borderline Psychopathology. Journal of Personality Disorders: Vol. 17, No. 3, pp. 233-242.
RESULT ON MODIFIED OVERT AGGRESSION SCALE (MOAS)
A similar analysis can be conducted for the MOAS test (Modified Overt Aggression Scale). The euthymic level (pertaining to a normal mood in which the range of emotions is neither depressed nor highly elevated) for MOAS is 0 to 2.
The initial mean MOAS scales were:
- Placebo group : 11.3
- Low dosage : 18.7
- Moderate dosage: 22.6
A strong decrease in verbal & physical aggression (MOAS) was noted. The following mean changes per week were noted in Table 4:
- – 0.37 for Placebo,
- – 1.92 for Low dosage,
- – 1.82 for Moderate dosage
Converting to changes over the 8 medication weeks of the trial, one gets total mean reductions of
- – 2.96 for Placebo
- – 15.36 for Low dosage
- – 14.56 for Moderate dosage
Hence the final mean MOAS scores were:
- Placebo : 8.34
- Low dosage : 3.34
- Moderate dosage: 8.04
So by the end of the study, the Low-dosage group was close to normal level on the overt aggression scale.
MOAS level for the Moderate-dosage started nearly 4 points higher than Low-dosage group. It achieved a similar reduction on the scale of about 15 points. The Moderate-dosage group h remained higher than normal for overt aggression (verbal or physical).
The Placebo group ended in the same range as the Moderate-dosage group, yet it started much lower. Hence the Placebo group did not progress nearly as much as either the Low-dosage or Moderate-dosage groups.
A similar series of effects were observed on the Young Mania Rating Scale (YMRS) for irritability, aggression and verbal outbursts, which uses total scores on a different scale. Yet the euthymic (normal) level for YMRS is also viewed as 0 to 2.
The initial mean YMRS scales were:
- Placebo : 3.6
- Low dosage : 3.3
- Moderate dosage : 4.4
A strong decrease in verbal & physical aggression (YMRS) was noted. The following mean changes per week were noted in Table 4:
- – 0.88 for Placebo
- – 2.08 for Low dosage
- – 2.40 for Moderate dosage
Hence the final mean MOAS scores were:
- – Placebo : 2.72
- – Low dosage : 1.22
- – Moderate dosage : 2.0
On this fine scale, it is clear the YMRS score of Low-dosage group reached the normal range, the Moderate-dosage group was just on the border, while the Placebo group remained in a disorder range.
Notes For Extra Care:
- These were mean scores. If we look at individual patients, one could surely see variations across individuals. We do not have this data to discuss here.
- The plateau reached at week 6 is interesting, and the fact that the Low dosage group continued going down at week 9 and 10. Is it medically significant, or too small an effect to mention?
- We do not know whether the effects of the medication were long lasting. Did the patients level on the scales change after stopping to take the medication? Within how many days or weeks after stopping the medication did their levels change. Taking the analogy of a medication that reduces the fever of a patient, the fever was closer to normal at the end of the trial. Did the fever remain low after they stopped taking medication, or did it go back up later on?
ZYPREXA (OLANZAPINE) STUDY WITH (DBT) TALK THERAPY FOR BPD, 2000-2002
“Olanzapine Plus Dialectical Behavior Therapy for Women With High Irritability Who Meet Criteria for Borderline Personality Disorder: A Double-Blind, Placebo-Controlled Pilot Study”, by Marsha Linehan, Joshua McDavid, Milton Brown, Jennifer Sayrs, and Robert Gallop. J. Clin. Psychiatry (June 2008) Vol. 69, No. 6, pp. 999-1005.
http://depts.washington.edu…/
The purpose of the study was to find out if the medication Olanzapine (commercial name: Zyprexa) reduced anger and hostility. The thought was that if patients’ emotions were less intense, they would benefit more from talk therapy.
This study ran from September 2000 to December 2002, and was performed with assistance from the University of Washington. Individuals were recruited from mental health clinics and by newspaper advertisements in the Seattle area.
For inclusion in the study, patients were required to be women aged 18 to 60 who met the following criteria:
- diagnosis of borderline personality disorder according to 2 structured interviews, the Personality Disorder Examination and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II);
- met borderline personality disorder criterion for inappropriate anger on the SCID II; and
- scored 6 or higher on the irritability subscale of the Overt Aggression Scale Modified for Outpatients (OAS-M).
Individuals were excluded if they:
- had a present diagnosis of schizophrenia, bipolar I disorder, schizoaffective disorder, major depressive disorder with psychotic features or other psychotic disorder, mental retardation or seizure disorder, or a diagnosis of substance dependence in the last 6 months according to the DSM-IV ;
- had an episode of self-inflicted injury (including a suicide attempt) in the 8 weeks prior to the screening interview; or
- were pregnant, breastfeeding, or planning to become pregnant.
Researchers found 24 borderline women satisfying those criteria of BPD with high levels of irritability and anger. They provided 21 weeks of Dialectical Behavior Therapy to these patients and split them randomly into two groups of 12 women. Individuals in one group received a low-dose Olanzapine (Zyprexa). The other group received a placebo.
At treatment start, all patients were given 1 tablet per day of study medication. Each tablet contained either 5 mg of olanzapine or placebo. After the first week of treatment, the daily dose was adjusted upward or downward in response to perceived response and side effects by 2.5 to 5 mg with an allowed dosage range of 2.5 to 15 mg per day.
Patients were also provided with Dialectical Behavioral Therapy (DBT) for the 21-week duration of the study.
At weeks 7, 14 and 21, the researchers measured symptoms of patients with standardized psychological measures (i.e. tests).
24 people is a relatively small group, yet it’s a start. 21 weeks is a bit less than 6 months which is a relatively long time, but that’s what it usually takes for DBT therapy to take effect.
This study experienced the following attrition. Eight patients (33%: 4 olanzapine, 4 placebo) dropped out of DBT and consequently were not continued on medication. Overall attrition rate in the study was 33%, leaving 67% of the subjects in the study till completion.
Five of those (21% of total) also dropped out of the assessment sequence before the final assessment; 4 (17%) missed the time-2 and time-3 assessments and 1 missed only the time-3 assessment.
One patient, assigned to the olanzapine condition, dropped out due to pregnancy (at week 10). In addition, 1 patient assigned to the olanzapine condition was removed from the study at week 7 due to psychotic symptoms (she was not counted as a drop- out).
Researchers concluded that “Olanzapine may promote more rapid reduction of irritability and aggression than placebo for highly irritable women with borderline personality disorder.”
DETAILED RESULTS OF THE 2000-2002 OLANZAPINE STUDY
Researchers found that both the people who received the medicine and the sugar pill both felt less irritable and hostile, and were less likely to self harm. However, the group that received the actual medication felt better more quickly. A notable reduction of several measurements of physical and verbal aggression was reported after 7 weeks and 14 weeks.
Overt Aggression Scale Modified for Outpatients (OAS-M) and Hamilton Rating Scale for Depression (HAM-D) were given at pretreatment (time 0) and at weeks 7 (time 1), 14 (time 2), and 21 (time 3) by clinical interviewers, naive to the subjects’ treatment conditions.
As can be seen on the chart below, the Olanzapine group decreased notably in physical and verbal aggression after just 7 weeks, while the Placebo group either remained about the same or went up in scores.
 For physical aggression: after 7 weeks, the Olanzapine group decreased from 5.5 before treatment to 2.0 at week 7, while the Placebo group online increased in physical aggression from 6.0 to 7.0. At week 14, the Placebook group decreased to 1.0 and caught up with the Olanzapine group still at 2.0. Then at week 21, the Placebo group went up to 2.0 while the Olanzapine group decreased to 0.0.
For physical aggression: after 7 weeks, the Olanzapine group decreased from 5.5 before treatment to 2.0 at week 7, while the Placebo group online increased in physical aggression from 6.0 to 7.0. At week 14, the Placebook group decreased to 1.0 and caught up with the Olanzapine group still at 2.0. Then at week 21, the Placebo group went up to 2.0 while the Olanzapine group decreased to 0.0.
For verbal aggression: after 7 weeks, the Olanzapine group decreased from 20.0 before treatment to 9.0 at week 7, while the Placebo group increased in physical aggression from 23.0 to 27.5. Then at week 14, the Olanzapine group further decreased to 4.0, while the Placebo group progressed to 15.5. Then at week 21, both groups were at level 9.0, which was a higher level for the Olanzapine group than at week 14.
For irritability and overt aggression: after 7 weeks, the Olanzapine group decreased from 7.0 before treatment to 5.0 at week 7, while the Placebo group did not change in score and stayed at 6.5. At week 14, the Olanzapine group stayed at level 5.0, while the Placebo group progressed to level 5.5. Then at week 21, both groups progressed, Olanzapine to level 3.5 and Placebo to level 4.5, hence Olanzapine ended up progressing slightly more (3 levels, from 6.5 down to 3.5) then Placebo (2.5 levels, from 7.0 to 4.5).
Scores for intentional self-injury fluctuated for both groups, toward a notable reduction. Both groups started at level 33.3. After 7 weeks, the Olanzapine group decreased to 16.7, while the Placebo group decreased to level 25.0. At week 14, the Olanzapine group decreased to level 12.5, while the Placebo group remarkably was at level 0.0. Then at week 21, both groups went back up 12.5 points, to reach level 25.0 for the Olanzapine group and level 12.5 for Placebo.
High suicidality was the one criteria where therapy did better than medication. Placebo started at level 50.0, then dropped to 9.3 at week 7, then 0 at week 14 and ended at level 12. 5 at week 21, while the Olanzapine group went from 41.7 at the start, down to 33.4 at week 7, up to 37.5 at week 14, and finally 25.0 at week 21.
Depression was more aided by medication than by therapy. On the Hamilton Rating Scale for Depression, the score of the Olanzapine group went 20.4 down to 12.6 at week 21, while the Placebo group went from 19.3 to 15.4 at week 21. The reduction in depression score also happened a bit faster at week 7 for the Olanzapine group.
Researchers concluded that “Analyses indicated that both treatment conditions resulted in significant improvement in irritability, aggression, depression, and self-inflicted injury. Irritability and aggression scores tended to decrease more quickly for the Olanzapine group than for the placebo group.
To sum up, the benefit of the Zyprexa medication compared to the Placebo was that people felt better more quickly. Both groups (real medicine vs. sugar pill) eventually experienced less anger and irritability.
Feeling better more quickly and lesser over aggression were the main benefit of medication seen here.
Considering the small size of this study, one could not say for sure that this finding was significant until we do more studies with a larger number of subjects and controls. The small sample size may have limited the ability to detect significant results.
The above study on Quetiapine (Seroquel XR) was a follow-on study focusing entirely on effects of medication for BPD.
All comments welcome. Please let us know what you think, especially if you have direct experience with medication and BPD
— by Frederic Bien, Ph.D.
President, PDAN (Personality Disorder Awareness Network)



If seroquel is the answer to my BPD in anyway why then does it make me pass out even after taking 25mg? I do not understand how this can be an adequate form of treatment if it leaves me drooling. I have accomplished a lot regardless of my diagnosis, but before I was able to reign in a lot of my own self destructive thoughts I landed myself in prison. Seroquel was the #1 thing the prison used to keep inmates at a comfortably numb state. They would come out for their meds breakfast, and you wouldn’t see them again until it was night med time. Then back to bed for them. I do not feel being medicated to the point of non existence is the answer for anyone. I am always looking for something that will help alleviate the aggression and anger outbursts, but have yet to find anything that may help. I do not want to lose the family that I have worked so hard to have. It took me 29 years to trust in another person that they maybe loved me enough to deal with me, and my constant insanity. I don’t want to lose that, but the thoughts are coming back of being free, running away, he hates me and really doesn’t love me, and the overall worst and most prevalent and reoccurring thoughts have to do with the children being out to get me, and intentionally doing things to hurt me. I am very self-aware and do know that these are irrational thoughts, but that does not excuse the internal anger and loathing that I have created for those around me based on actions that are of normal dis-meaner for any sane individual. What meds really work. My Dr. literally has given up as I have been medicated since I was 5 due to my mother feeling her degree in behavioral health nursing automatically meant she could asses and diagnose me as crazy at 5, and somehow get me medicated from Ritalin at 5 to lithium by age 10 to putting me in the hospital at 12 for bulimia (which I NEVER had mind you. I just had to go to the bathroom during meals), now being made to feel crazy is a HUGE trigger for me. I just want to be better and know what I can take to do this. I recently switched counselors after 12 years as she was not doing anything, and I am hoping for more dialect between us to reach further results. I am also working on a book of my own as I have often led a life of more fiction than reality. Those that have lived it with me or watched it happen are sometimes some of the only people that truly know that some of the stories are very true. I just want to be happy now though. I am tired of feeling like I need to run and be free, or have sex with who ever because I can, or keep turning myself into an alcoholic, even ruining my relationship due to my inability to maintain a stable emotion for more than what could be a few hours. I’m tired of feeling empty to the point of nothingness, and trying to explain to my partner how that truly feels. I hate when he is hugging me and I am staring off in the distance with no emotion of any kind going on inside of me, or even hugging my own children and there is just nothing. No love, no hate, no nothing of any kind. I am just void of emotions, and when they do come in more often than not they are of seething anger. I am as rational as I can be for everything I have been through, but if there are other meds that may work I would love to know. I am allergic to welbuterin, paxil, amatriptilyne, and do not tolerate any kind of NSAID due to stomach surgeries. I have fibro and have literally tried every FDA approved med possible. I have been diagnosed with BPD, Bipolar, ADHD, ODD (at a younger age. I don’t feel that really applies now.), Anxiety, and Depression. I have tried so many meds that have not worked it has caused my head dr as I refer to her as to give up on trying anything different anymore, so I am on xanex 1mg (which I hate. I cant even take 1 and I pass out and that is after over a year), and I am on Vyvanse 60mg which does seem to reign in on the randomness of everything going on in my head for the most part. That is all I get from my head Dr. My regular dr has me on my pain meds for fibro and bp meds due to a “LITERALLY” unbelievable pregnancy from almost 3 years ago already. The dr I had that left me shortly after I gave birth was very right in her statement of “if it can it will!” that was her motto with me she called me her medical anomaly… There is too much going on inside of me, and I don’t know how long it will take for whatever it is to either kill me from an autoimmune standpoint, or what else. I don’t ever want to try and kill myself again that is not what I was getting at but the fact remains that there are times when I cannot guarantee my own safety (as rare as they may be) there are still times that my brain is trying to make me feel like “every one would be better off without me!” or that I am literally a burden on everyone around me, and so many more. It is a constant battle to convince myself that I AM worthy and that what I am feeling is not true, or that it is not how I truly feel when I am capable of the full extent of my emotions. Well now that I have written this short book for you I really hope that someone, anyone can give me some sort of advice as to what to do. It is hard enough dealing with what I feel is being crazy, and it makes it even harder living 40 miles from the nearest hospital, psychiatric dr, or even pharmacy. If nothing else I would like to not become some sort of messed up statistic for those that were not able to receive the help that they needed. I know I need help and I am more than wiling to get it if it is offered to me. Thank you to whomever may read this, and offer me some sort of relief to the struggles that I find myself going through struggling with this horrible diagnosis. Which PS has been since I was a teenager. Thank you for reading.
I suffer also from emotional turbulence and intensity syndrome (BPD). I have found that eating a vegetarian diet helps decrease my aggression intensity, very significantly. Best wishes to you. You are a champion.
Hi Nikki,
Hope You got your meds, support and wellbeing sorted. And I really hope you told your head Dr. about the quetiapine making you too drowsy. Your sensitivity to medication limits your choices BUT quetiapine comes in different formulation. There is a less sedating one but with effective mood stabilizing effect. You doctor can help
I am going through almost everything you have described. I am 32, and realized that I had some sort of serious personality disorder around 28. I knew I was messed up around 14 but I did not know what kind of ride I was in for until I looked at my actions and my role in society over the last 16 years. I was also diagnosed with ADHD at 5 and put on ritalin, diagnosed with social anxiety disorder and depression at 18. After spending day and night reading about personality disorders, although your not supposed to self diagnose, I am convinced I have BPD and possibly some other disorders.. I am finding that as I get older and continue read up on this terrible affliction I am starting to get some sense of control. As small as a grain of sand but I feel like I am moving in the right direction. The first changes began when I started to evaluate my behaviour and how I have hurt so many people and I did not feel ashamed the way a normal person would. I also could convince myself that I was a good person who was just misunderstood. I always took the bad things and said in some form, that is not me, I only did that because I had to. This thinking of I am special and am above all the rules was rooted in my head deep. The biggest change for me is always being mindful of other people and not being selfish. Now that I am making these changes, sometimes I am appalled by how much of a sociopath I was. Before I found out what BPD was I was almost proud that I was “so smart and able to get away with things while others have to follow the rules” While thinking at the same time I am such a nice person. It scares me now to know that I could be such a person and that its still in there lurking if I do not use preventative measures every day, every hour, every minute. Everyone thought of me as a nice person but deep down I knew the truth and that is what triggered the incredible sense of self loathing. I thought there that anyone who wanted to hang out with a person like me is not someone I want to be around. This would cause me to act out in odd ways and abuse alcohol constantly because I also have extreme social anxiety. Now I try to push myself even in the smallest steps towards putting others ahead of me and having real lasting feelings for the pain of others, and asking if there is something I can do to help. I do not always act on these positive thoughts but atleast I do not reward myself the way I use to for just thinking about doing a good deed. I had a bad habit of that, thinking just because I thought about doing the right thing it counted as having done it. It does not, and for me it’s not positive even in a moral sense, it is devious and destructive because it triggers the worst part of my BPD. It has been a hard pill to swallow but when I get to thinking how bad I feel, I redirect that into thinking about all the people I hurt on a continuous basis, and it was almost always the people who cared about me the most. As far as I am concerned I am no longer allowed to feel sorry for myself, if I want to feel sorry I should feel sorry for the person I was and hope that person has been transformed. I use to struggle with believing in God, and I wanted to but the logic never added up in my head. It hit me like a ton of bricks when I realized that after all the deeds I have done and never paid the price, I better hope there is not a God. I started praying, because suddenly I believed and it was at a hard time to make the switch, because I just knew I belonged in hell. It does not take a huge leap to change, even taking baby steps, the smallest things add up. Continue to persevere and strive to think of others, there are people suffering far worse out there. No matter how small, even if it feels pointless, do something different. Do the right thing that you know other people would also validate as the right thing. It is like moving tiny grains of sand one at a time, its hard but what choice is there other than watch the pit of agony get worse and worse with age. Keep a close eye on your emotions and think things through, it will get easier with time, our brains have the ability to rewire and adapt but it takes time, and it’s very hard for me to wait. I am extremely impulsive and want results immediately in almost everything I do. So this is also a lesson in patience.
Hi I just read your post and I want to say thank you thank you . I have not heard a person with a personality disorder be so self reflective and humble ( that’s part of the disease) also I applaud your effort and hard work !!!! Again goes against the disorder . My mother had a personality disorder ( some NPD) and it was hell on me growing up and still is a challenge a very difficult challenge because as you said IT HURTS soooo many people . But I have also grown in my own strength and faith in God as a result . Also glad you are now seeking a loving and forgiving God – may you continue to do so . Thank you again for your post and may I make a suggest WRITE a book. There are many out there that need to know this dis-ease can be overcome it would bless many families because everyone suffers ! Lastly I wrote a book a memoir . It’s my life story living with a mom who has a personality disorder and how it chsnged the terjectory of my life , it’s cslled “How I Broke The Generational Curse”. Oh one last thing Joyce Meyers excellent author motivational speaker and godly woman documents her victory over self loathing
Be well
Hi Dustin
Just wanted to say hang in there.
Jeff
My God, this wasn’t written by me?!?! Is there hope? Even though suicide is repugnant to me because Anthony Bordian lead me to know my pain is not, is never, more important than the pain my death might cause someone else (although my disease lets me know some would be relieved). But sometimes the pain and the shame are overwhelming. I don’t want to kill myself or have someone else like the police do it for me, but sometimes I just don’t want to be alive. I try to think if I could use my time left to ameliorate some of the damage I’ve done, but in my pain I usually make things worse. Therapy hasn’t helped me change so far, but I am going to an outpatient clinic concentrated course later this month. I pray it helps. I can’t take much more.
Hi Dotty,
Don’t give up. It is hard and can get harder or easier. You have so much strength to assert that you want to keep yourself safe even if you don’t feel like being alive sometimes. Different things work for different people. Hope the program works.
Strength
Hello my name is Tania I came across your site on facebook but I’m no longer using facebook. But I have gotten emails from your site. I’ve just read the article on the new trails u were running for people like myself I suffer terrible with this disorder. I live in Perth western Australia and there were not trips of these drugs for myself r u u able to help me got on this tril or start it up over here with my doctor. It just could help me and many others here in Perth I take that many medicine to keep it hidden for people as they say I’m crazy mad sicot. And a lot of them want to lock me up in a mental hospital. Even my ex boyfriend whom I’m now seeing him and he really understanding of my illness but. I can do this any mire
Diagnosed first in 1089 inpatient.
What is the medical treatment for BPD
Hi I’m going through some of the things you have described. I am now 61 yrs old & I’m starting to realize that i am going through some personality disorder. It started 4 yrs ago at 57. It was a very emotional traumatic experience in my life with my family. I started counseling 4 yrs ago which is helping thank God ! At the present time I’m still hurt & feeling that i don’t belong to my family as much as i was 4 yrs ago. All 3 of them were my life (tears) i hurt & cry alot on a daily basis for 4 yrs. I take my feelings out on my partner whom i truly love & it’s not right.I just feel like nobody gets it. Do i fall in this category of BPD please help me to figure this out. Thank you.
My doctor told me to look this up .